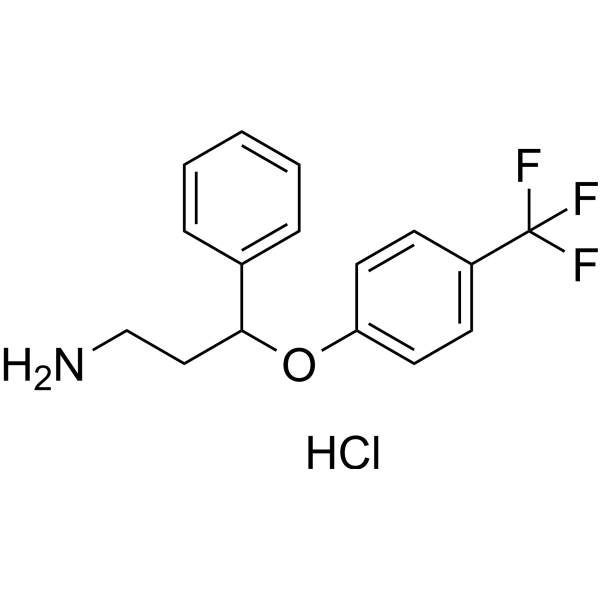
Norfluoxetine Hydrochloride
CAS No. 57226-68-3
Norfluoxetine Hydrochloride( Norfluoxetine HCl )
Catalog No. M27362 CAS No. 57226-68-3
Norfluoxetine hydrochloride is an active metabolite of fluoxetine. Fluoxetine is an antidepressant drug.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 149 | Get Quote |


|
| 10MG | 217 | Get Quote |


|
| 25MG | 357 | Get Quote |


|
| 50MG | 475 | Get Quote |


|
| 100MG | 689 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameNorfluoxetine Hydrochloride
-
NoteResearch use only, not for human use.
-
Brief DescriptionNorfluoxetine hydrochloride is an active metabolite of fluoxetine. Fluoxetine is an antidepressant drug.
-
DescriptionNorfluoxetine hydrochloride is an active metabolite of fluoxetine. Fluoxetine is an antidepressant drug.
-
In Vitro——
-
In VivoPretreatment with Fluoxetine or Norfluoxetine hydrochloride (20mg/kg s.c.), as well as Phenytoin (30 mg/kg s.c.) and Clonazepam (0.1mg/kg s.c.) significantly increases both the rate and duration of survival, demonstrating a significant protective effect against Pentylenetetrazol-induced epilepsy.
-
SynonymsNorfluoxetine HCl
-
PathwayOthers
-
TargetOther Targets
-
Recptortyrosine hydroxylase (TH)
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number57226-68-3
-
Formula Weight331.76
-
Molecular FormulaC16H17ClF3NO
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 125 mg/mL (376.78 mM)
-
SMILESCl.NCCC(Oc1ccc(cc1)C(F)(F)F)c1ccccc1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Luck LA, et al. Fluorescence and 19F NMR evidence that phenylalanine, 3-L-fluorophenylalanine and 4-L-fluorophenylalanine bind to the L-leucine specific receptor of Escherichia coli. Protein Sci. 2000;9(12):2573-2576.
molnova catalog



related products
-
[Des-octanoyl]-Ghrel...
Non-acylated, major circulating isoform of ghrelin
-
Diethylene glycol bi...
Diethylene glycol bis(p-toluenesulfonate) is a PEG-based PROTAC linker that can be used in the synthesis of PROTACs.
-
SGA360
SGA360 is a selective aryl hydrocarbon (Ah) receptor modulator. It exhibits anti-inflammatory properties.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


